Vero Cell Covid Vaccine Efficacy Rate : Who Approves China S Sinopharm Covid 19 Vaccine For Emergency Use Has 79 Efficacy Coronavirus Outbreak News
Efficacy at preventing infection. Phase 3 trials of Sinopharms vaccine showed an efficacy rate of 79 per cent against symptomatic Covid infection if the shots were administered three weeks apart.

Who Approves China S Sinopharm Covid 19 Vaccine For Emergency Use Has 79 Efficacy Coronavirus Outbreak News
This happens because it is the most visible phrase that is not written in Chinese on the packaging.
Vero cell covid vaccine efficacy rate. So far Vero is the only Chinese vaccine for which the manufacturer has published official data. Estimating vaccine efficacy for COVID-19 projections. It is also found as COVID-19 BIBP vaccine or simply Sinopharm vaccine.
COVID-19 Vaccine Vero Cell Inactivated detailed edition Title. EMA has initiated rolling reviews for the following COVID19 vaccines. Assessments in settings where the P2 Variant of Concern was widely circulating also in Brazil - estimated vaccine effectiveness of 496 following at least one dose and demonstrated 507 two weeks after the second dose.
Efficacy at preventing symptomatic disease. NVX-CoV2373 developed by Novavax rolling review started on 3 February 2021 CVnCoV by Curevac rolling review started on 12 February 2021 Sputnik V Gam-COVID-Vac by Gamaleya rolling review started on 4 March 2021 and COVID-19 Vaccine Vero Cell Inactivated by Sinovac Life Sciences Co Ltd rolling review started. Historically it is the first cell line that was approved by the WHO for the production of human vaccines.
Efficacy Safety and Immunogenicity of Inactivated SARS-CoV-2 Vaccines Vero Cell to Prevent COVID-19 in Healthy Adult Population In Peru Healthy Adult Population In Peru Covid-Peru The safety and scientific validity of this study is the responsibility of the study sponsor and investigators. Interval of 21 days had the efficacy of 79 against symptomatic SARS-CoV-2 infection 14 days or more after the second dose. The secondary objectives include evaluating the efficacy after at least one dose of the vaccine against RT-PCR confirmed symptomatic.
What is the effectiveness of two doses of inactivated Vero cell-derived JE vaccine in preventing JE disease in individuals living in JE-endemic areas. The Sinopharm vaccine is less effective than those developed by Pfizer-BioNTech and Moderna which have an efficacy rate of about 95. A Study to Evaluate The Efficacy Safety and Immunogenicity of Inactivated SARS-CoV-2 Vaccines Vero Cell in Healthy Population Aged 18 Years Old and Above COVID-19 The safety and scientific validity of this study is the responsibility of the study sponsor and investigators.
SARS-CoV-2 exhibits extremely efficient human-to-human transmission the new pathogen rapidly spread worldwide and within months it caused a global pandemic changing daily life for billions of people. Efficacy against COVID-19 cases in Turkey Treatment Arm Number of symptomatic COVID-19 Cases Total Subject Number Person year exposure Incidance rate 100 person year CoronaVac 9 6559 014 28389 317 Placebo 32 3471 092 16653 1922 Total 41 10030 Vaccine Efficacy 8350 CI 95 6542-9212. Vero cell vaccine covid-19 efficacy rate.
Vero cell vaccine covid-19 effectiveness. Efficacy Safety and Immunogenicity of Inactivated SARS-CoV-2 Vaccines Vero Cell to Prevent COVID-19 in Healthy Adult Population In Peru Healthy Adult Population In Peru Covid-Peru. The primary objective is to evaluate the efficacy of an inactivated and aluminium hydroxide adsorbed SARS-CoV-2 vaccine Sinovac China in voluntary participants after 14 days of the second dose against RT-PCR confirmed symptomatic COVID-19 cases.
This is operationalized as a reduction in. The Sinopharm product is an inactivated vaccine called SARS-CoV-2 Vaccine Vero Cell. Sinopharm announced on Wednesday that its Beijing vaccine was 79 effective.
Efficacy Safety and Immunogenicity of Inactivated SARS-CoV-2 Vaccines Vero Cell to Prevent COVID-19 in Healthy Adult Population In Peru Healthy Adult Population In Peru - Full Text View. The Cuban government announced on June 22 2021 that its three-shot Abdala vaccine has a 92 efficacy rate against COVID-19. Currently the IHME model uses the following inputs of vaccine efficacy separated by variant.
As new data becomes available WHO. Sinopharm vero cell vaccine efficacy. The findings were reported in the May 26 Journal of the American Medical Association.
A leading US medical journal found that the two vaccines prevented symptomatic infections by 728 per cent and 781 per cent largely in line with what the state-owned drug maker previously announced. A large multi-country Phase 3 trial has shown that 2 doses administered at an interval of 21 days have an efficacy of 79 against symptomatic SARS-CoV-2 infection 14 or more days after the second dose. The COVID-19 case fatality rate of 25 makes.
A large phase 3 trial in Brazil showed that two doses administered at an interval of 14 days had an efficacy of 51 against symptomatic SARS-CoV-2 infection 100 against severe COVID-19 and 100 against hospitalization starting 14 days after receiving the second dose. Vero cell vaccine covid-19 efficacy rate. Vero cell covid vaccine efficacy rate.
Vaccine efficacy against laboratory-confirmed symptomatic COVID-19 was estimated to be 78 in adults 18-59 years of age. Sinopharm vero cell vaccine efficacy. Actual Study Start Date.
On December 29 2020 Sinopharm reported 79 efficacy in an interim evaluation. This is operationalized as a reduction in hospitalization and death. However the immunogenicity and efficacy of traditional egg-derived inactivated trivalent influenza vaccines.

Interim Estimates Of Vaccine Effectiveness Of Bnt162b2 And Mrna 1273 Covid 19 Vaccines In Preventing Sars Cov 2 Infection Among Health Care Personnel First Responders And Other Essential And Frontline Workers Eight U S Locations December
Sinopharm S Two Covid 19 Shots Effective Study Says Reuters

Integrated Control Of Covid 19 In Resource Poor Countries International Journal Of Infectious Diseases

Seychelles Brings Back Curbs Despite Vaccination Success Bbc News

China S Covid Vaccine From Sinopharm Is 86 Effective Uae Says
Https Cdn Who Int Media Docs Default Source Immunization Sage 2021 April 1 Sage29apr2021 Sinopharm Pdf Sfvrsn Ddf0d841 5
Peru Study Finds Sinopharm Covid Vaccine 50 4 Effective Against Infections Dhaka Tribune
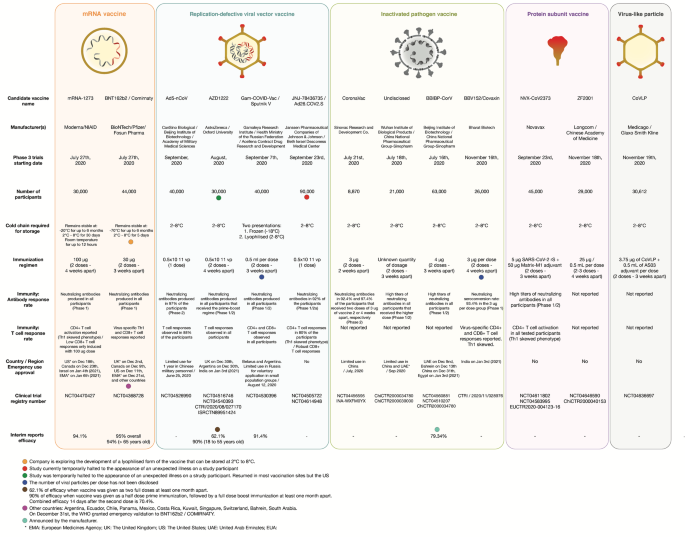
Sars Cov 2 Vaccines Strategies A Comprehensive Review Of Phase 3 Candidates Npj Vaccines

Who Lists Sinopharm S Covid 19 Vaccine For Emergency Use
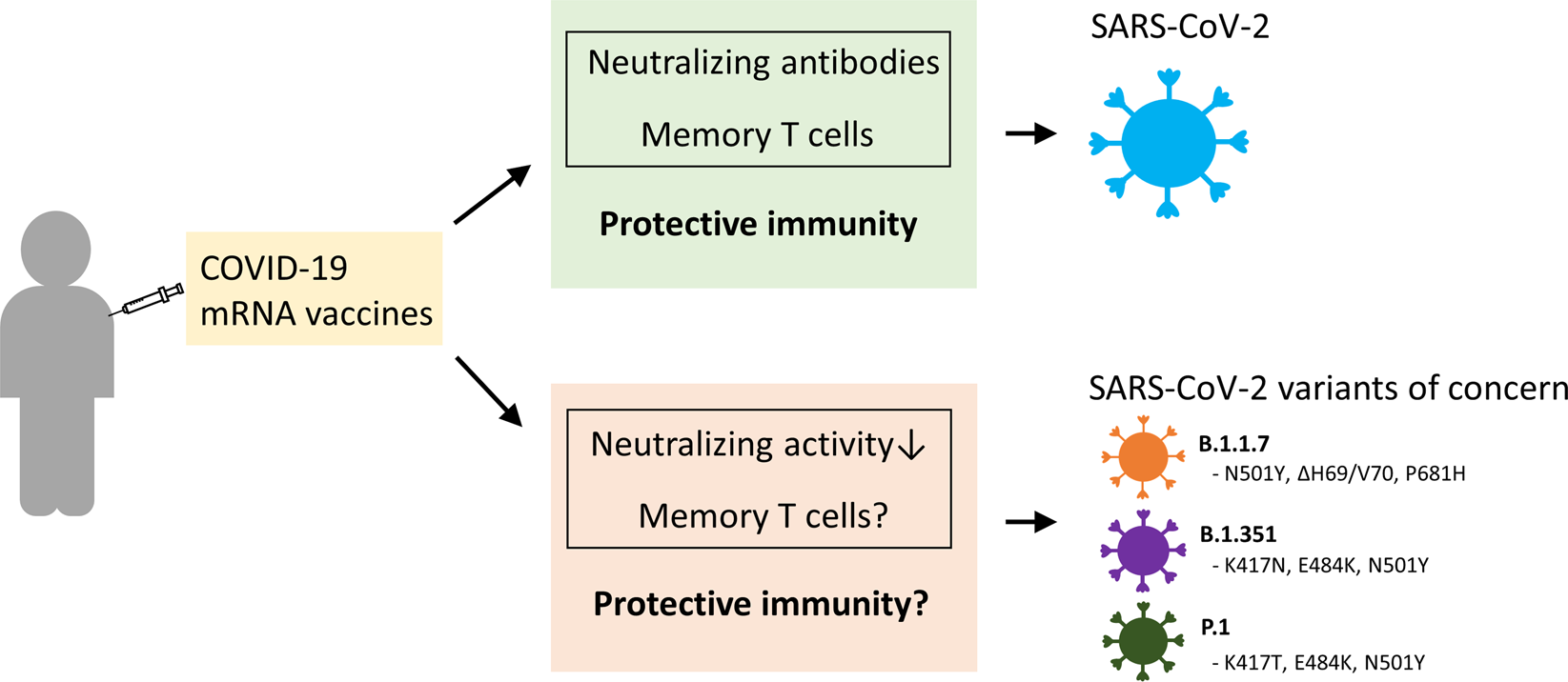
Sars Cov 2 Mutations Vaccines And Immunity Implication Of Variants Of Concern Signal Transduction And Targeted Therapy

Efficacy Of Sinopharm S Covid 19 Vaccines Proved Again In New Trials Cgtn
Https Cdn Who Int Media Docs Default Source Immunization Sage 2021 April 1 Sage29apr2021 Sinopharm Pdf Sfvrsn Ddf0d841 5
Covid Bolsonaro Hails Suspension Of Chinese Vaccine Trial Bbc News
Coronavirus Covaxin Efficacy Is 81 Works Against Variants The Hindu

Safety Tolerability And Immunogenicity Of An Inactivated Sars Cov 2 Vaccine In Healthy Adults Aged 18 59 Years A Randomised Double Blind Placebo Controlled Phase 1 2 Clinical Trial The Lancet Infectious Diseases
Sinopharm S Covid 19 Vaccine Shows 86 Efficacy Uae Health Agency Says Biospace
Uae Says Sinopharm Vaccine Has 86 Efficacy Against Covid 19 Reuters

Sinopharm Vero Cell Inactivated Covid 19 Vaccine

The Science Behind Chinese Covid 19 Sinopharm Vaccine Inactivated Virus And Mystery Of Falling Immunity Science News
