Vero Cell Covid Vaccine Efficacy Percentage : Who Approves China S Sinopharm Covid 19 Vaccine For Emergency Use Has 79 Efficacy Coronavirus Outbreak News
This is operationalized as a reduction in susceptibility. The percentage of patients with non-Hodgkin lymphoma who were seronegative following vaccination ranged from 21 to 56.
Coronavirus Covaxin Efficacy Is 81 Works Against Variants The Hindu
While EMA cannot predict the overall timelines it should take less time than normal to evaluate an eventual application because of the work done during the rolling review.
Vero cell covid vaccine efficacy percentage. Vaccine efficacy against hospitalization was 79. Both the Wuhan and the Beijing vaccines use the inactivated virus cultivated in vero cells or kidney epithelial cells from the African Green Monkey. Inactivated SARS-CoV-2 vaccine Vero cell Inactivated SARS-CoV-2.
How efficacious is the vaccine. Many of these patients were treated with anti. On December 29 2020 Sinopharm reported 79.
A large phase 3 trial in Brazil showed that two doses administered at an interval of 14 days had an efficacy of 51 against symptomatic SARS-CoV-2 infection 100 against severe COVID-19 and 100 against hospitalization starting 14 days after receiving the second dose. Adjuvanted inactivated vaccine produced in Vero cells and developed by India showed 81 efficacy in preventing COVID-19 after two doses. EMA will assess the compliance of COVID-19 Vaccine Vero Cell Inactivated with the usual EU standards for effectiveness safety and quality.
Meanwhile Sinovac previously said its vaccine was 50 effective which the Department. A large multi-country Phase 3 trial has shown that 2 doses administered at an interval of 21 days have an efficacy of 79 against symptomatic SARS-CoV-2 infection 14 or more days after the second dose. Efficacy at preventing infection.
Estimating vaccine efficacy for COVID-19 projections. At present our model only distinguishes. On the basis of all available evidence WHO recommends the vaccine for adults 18 years and older in a two-dose schedule with a spacing of three to four weeks.
Referring to the above principles the SARS-CoV-2 inactivated vaccine Vero cells was administered once each on days 0 14 28 and 42 for a total of four administrations and the interval was 2 weeks followed by a recovery period after the last administration of 2 weeks 14 days. Vaccine efficacy for symptomatic and hospitalized disease was estimated to be 79 all age groups combined. The efficacy rating followed an interim report of ongoing human trials conducted in that country CNBC reported.
Efficacy Safety and Immunogenicity of Inactivated SARS-CoV-2 Vaccines Vero Cell to Prevent COVID-19 in Healthy Adult Population In Peru Healthy Adult Population In Peru Covid-Peru The safety and scientific validity of this study is the responsibility of the study sponsor and investigators. Efficacy at preventing symptomatic disease. Inactivated SARS-CoV-2 vaccine Vero cell Inactivated SARS-CoV-2.
Chinas Sinopharm says vaccine 79 effective against COVID-19. Dec 09 2020 By Alex Keown The COVID-19 vaccine under development by Chinas Sinopharm is showing efficacy of 86 health authorities from the United Arab Emirates reported this morning. Sinopharm announced on Wednesday that its Beijing vaccine was 79 effective.
Vaccine Efficacy 8350 CI 95 6542-9212 Based on symptomatic and RT-PCR positive COVID-19 cases after 14 days and more after the 2nd dose Number of subjects after 14 days and more after the 2nd dose Based on calculation person x year in the follow up period Treatment Arm Number of hospitalized COVID-19 Cases Total Subject. Phase 3 ChiCTR2000034780 ChiCTR2000039000. Phase 3 ChiCTR2000034780 NCT04560881.
Few adults over 60 years were enrolled in clinical trials so efficacy could not be estimated in this age group. COVID-19 Vaccine Vero Cell Inactivated detailed edition Created Date. Data so far suggests efficacy rates of approximately 67 percent for the JJ vaccine 66 to 95 percent for the Moderna vaccine and 42 to 96 percent for the Pfizer-BioNTech vaccine.
Vaccines were shown to have 9495 efficacy in preventing symptomatic COVID-19 calculated as 100 1 minus the attack rate with vaccine divided by the attack rate with placebo. In contrast just one of 64 Hodgkin lymphoma patients was seronegative. Participants will be randomly inoculated with two doses of investigational vaccine or placebo according to 11 ratio following Day 0-Day 14 immunization schedule and will be observed from the first dose of investigational vaccine to collect symptomatic and laboratory-confirmed COVID-19 cases for the evaluation of the efficacy of the investigational vaccine.
QazCovid-in - COVID-19 inactivated vaccine. This is operationalized as a reduction in hospitalization and death. Currently the IHME model uses the following inputs of vaccine efficacy separated by variant.
Listing a study does not mean it has been evaluated by the US. Serbia looks east to fill coronavirus vaccine shortage So far Vero is the only Chinese vaccine for which the manufacturer has published official data. Vaccine efficacy for symptomatic and hospitalised disease was estimated to be 79 percent all age groups combined.
This includes patients with diffuse large B cell mantle cell marginal zone and follicular lymphomas as well as Waldenstroms macroglobulinemia.
World Health Organization Who What S The Difference Between Covid 19 Vaccine Efficacy And Effectiveness Vaccine Efficacy Refers To How The Vaccine Performs In Ideal Conditions Controlled Clinical Trials Vaccine
Sinopharm S Covid 19 Vaccine Shows 86 Efficacy Uae Health Agency Says Biospace
Https Cdn Who Int Media Docs Default Source Immunization Sage 2021 April 1 Sage29apr2021 Sinopharm Pdf Sfvrsn Ddf0d841 5

Safety Tolerability And Immunogenicity Of An Inactivated Sars Cov 2 Vaccine In Healthy Adults Aged 18 59 Years A Randomised Double Blind Placebo Controlled Phase 1 2 Clinical Trial The Lancet Infectious Diseases

Who Lists Sinopharm S Covid 19 Vaccine For Emergency Use
Https Cdn Who Int Media Docs Default Source Immunization Sage 2021 April 1 Sage29apr2021 Sinopharm Pdf Sfvrsn Ddf0d841 5

Who Approves China S Sinopharm Covid 19 Vaccine For Emergency Use Has 79 Efficacy Coronavirus Outbreak News

Interim Estimates Of Vaccine Effectiveness Of Bnt162b2 And Mrna 1273 Covid 19 Vaccines In Preventing Sars Cov 2 Infection Among Health Care Personnel First Responders And Other Essential And Frontline Workers Eight U S Locations December

Seychelles Brings Back Curbs Despite Vaccination Success Bbc News
Who Approves Sinopharm Vaccine In Potential Boost To Covax Pipeline Reuters

Efficacy Of Sinopharm S Covid 19 Vaccines Proved Again In New Trials Cgtn

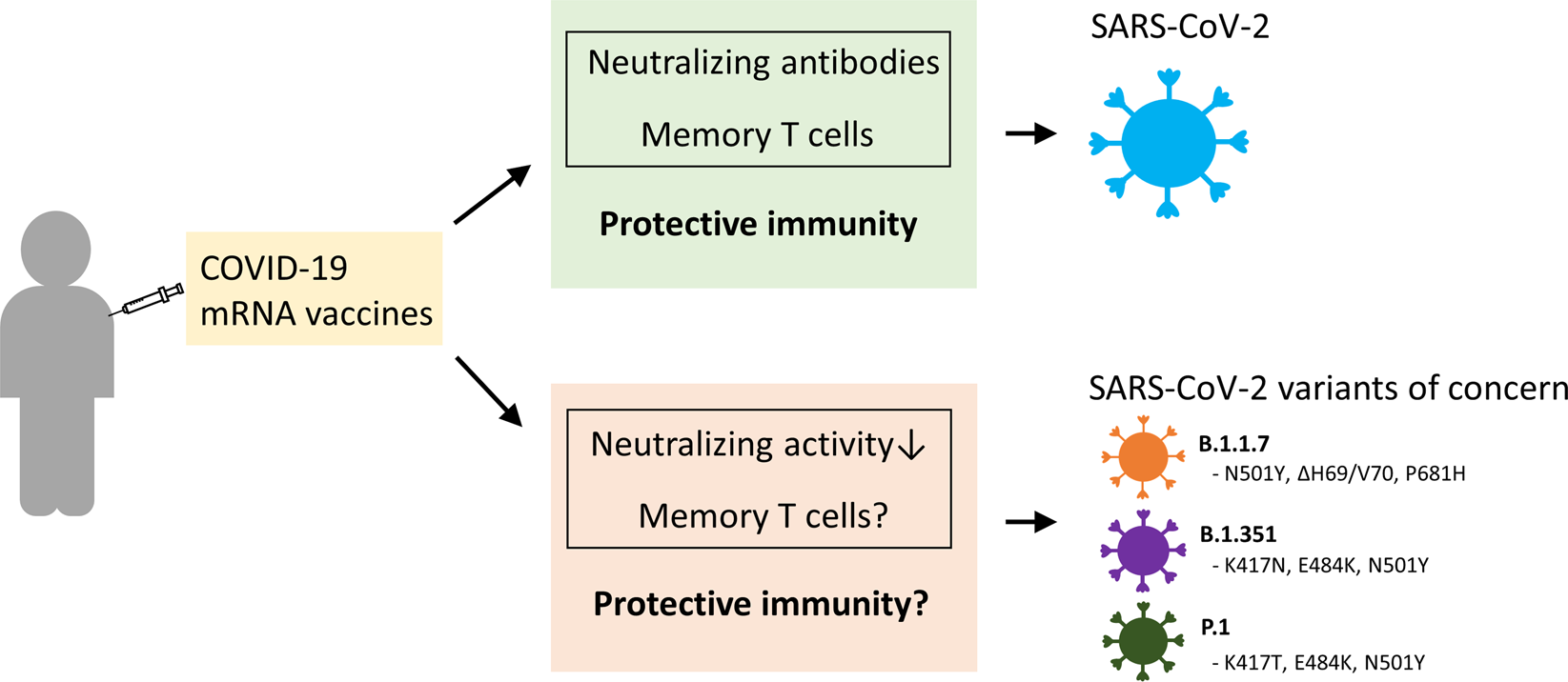
Sars Cov 2 Mutations Vaccines And Immunity Implication Of Variants Of Concern Signal Transduction And Targeted Therapy
Https Cdn Who Int Media Docs Default Source Immunization Sage 2021 April 1 Sage29apr2021 Sinopharm Pdf Sfvrsn Ddf0d841 5

Coronavirus Who Approves Sinovac Covid Vaccine For Emergency Use News Dw 01 06 2021

Efficacy And Safety Of An Inactivated Whole Virion Sars Cov 2 Vaccine Coronavac Interim Results Of A Double Blind Randomised Placebo Controlled Phase 3 Trial In Turkey The Lancet

Sinopharm Vero Cell Inactivated Covid 19 Vaccine
Sinopharm S Two Covid 19 Shots Effective Study Says Reuters
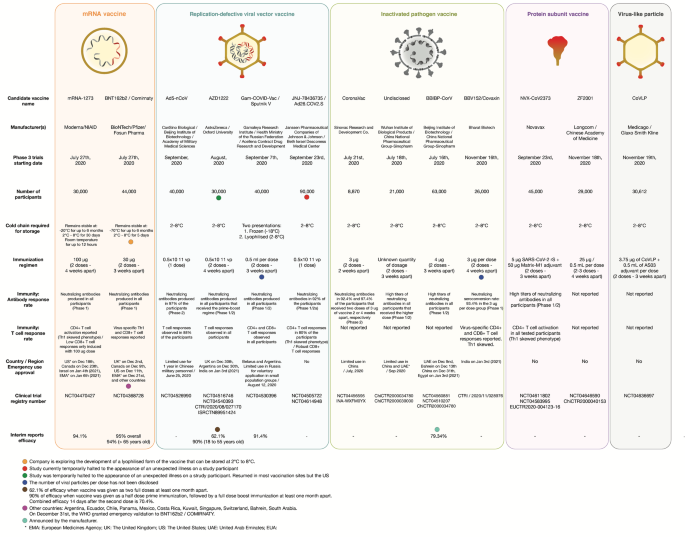
Sars Cov 2 Vaccines Strategies A Comprehensive Review Of Phase 3 Candidates Npj Vaccines

China S Covid Vaccine From Sinopharm Is 86 Effective Uae Says
